Catalog of Regulatory Science Tools to Help Assess New Medical Devices
4.6 (301) · € 37.99 · Auf Lager


MS in Regulatory Science
Science and Engineering Laboratories: Updates from FDA/CDRH - US FDA

Emerging Telemedicine Tools for Remote COVID-19 Diagnosis, Monitoring, and Management
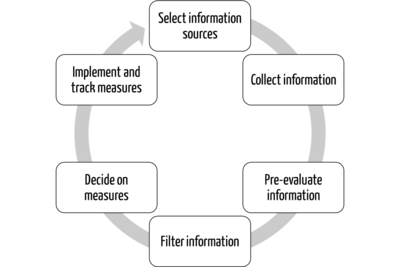
Regulatory Intelligence - a core task of Regulatory Affairs?
FDA Updates Catalog of Tools to Assess Medical Devices - Medical Design Briefs

Safety & Regulatory requirements for Medical Devices - APCER Life Sciences
Updates to the Catalog of Regulatory Science Tools to Help Assess New Medical Devices - US FDA

Regulatory Review of Novel Therapeutics — Comparison of Three Regulatory Agencies
How FDA Regulates Artificial Intelligence in Medical Products
Latest CDRH Updates: Extended Shelf Life for At-Home COVID-19 Tests & More - US FDA
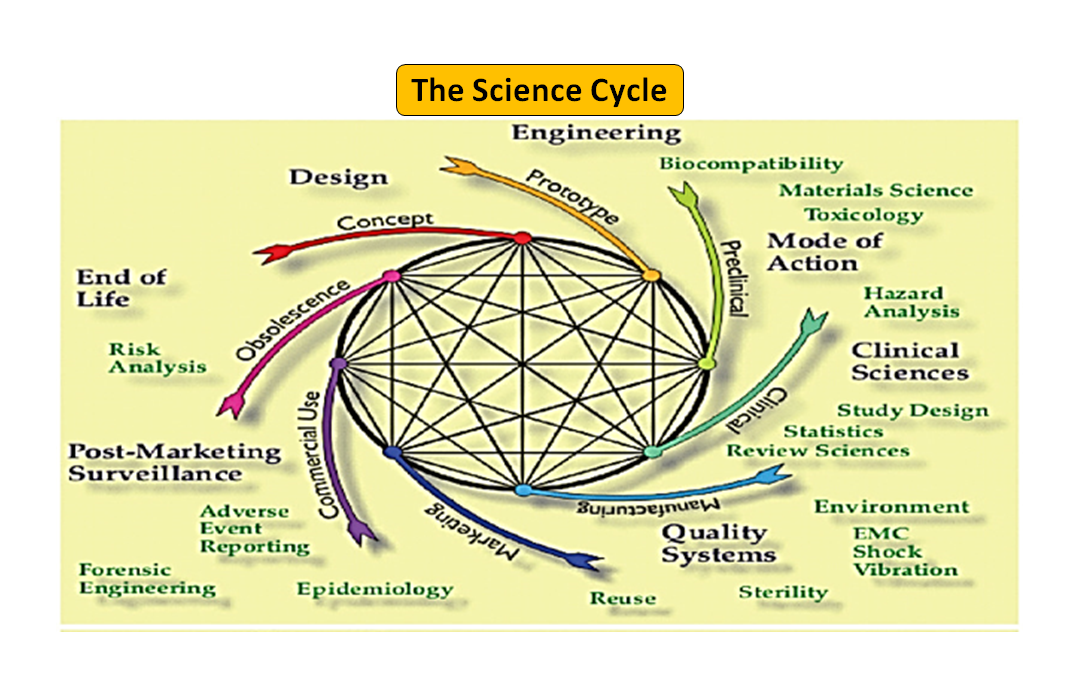
Medical Device Design: The Essential, Step-by-Step Guide

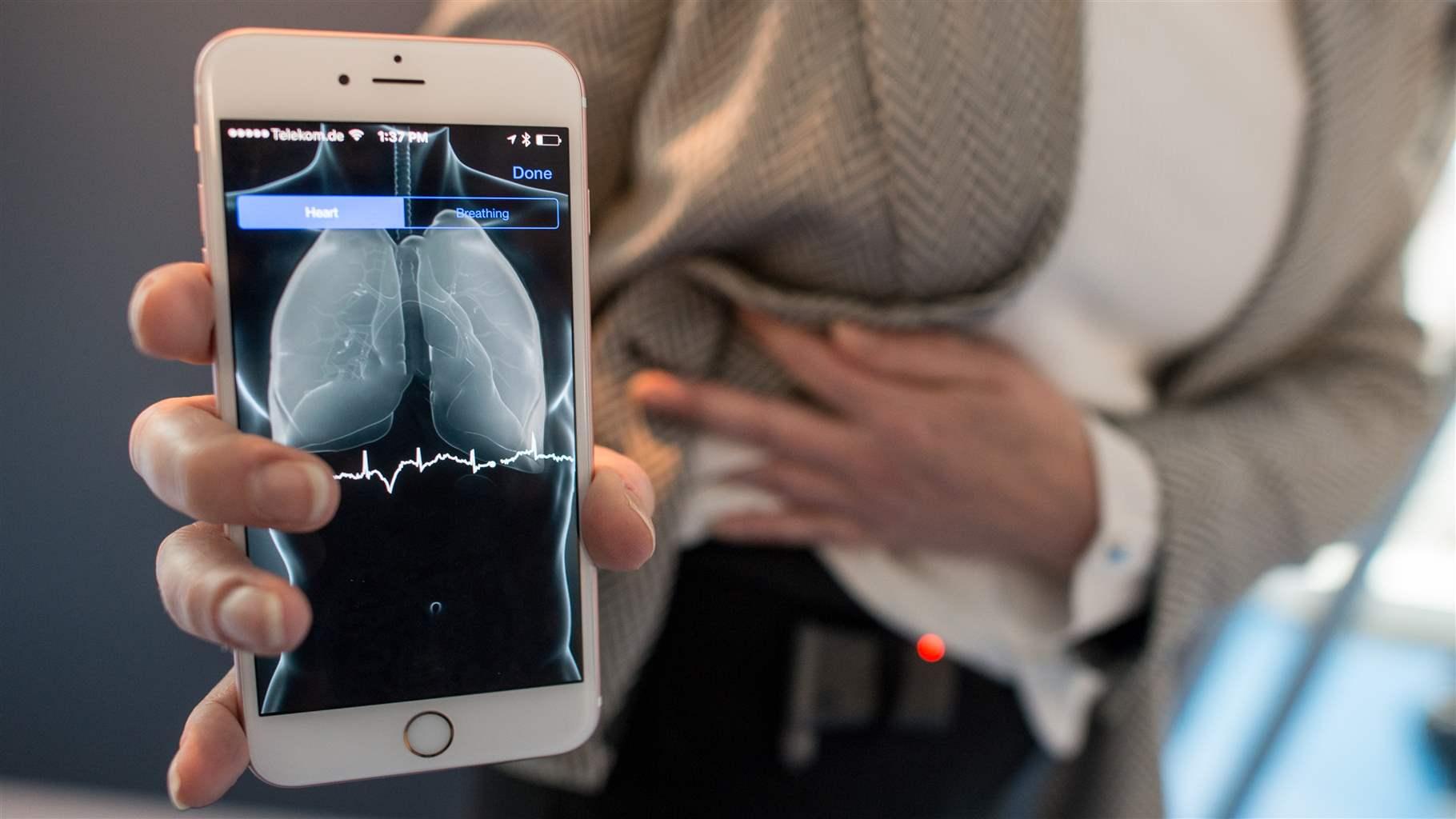
Your Medical Devices Are Getting Smarter. Can the FDA Keep Them Safe? - WSJ

Interdisciplinary Academic Framework for Medical Regulatory Science|What is RS?|Institute for Medical Regulatory Science
동국대학교 바이오헬스의료기기규제과학과

New IAEA Methodology Offers Remote and Automated Quality Control of Radiography and Mammography Equipment